Genetically Modified Biological Heart Valves
Worldwide there are about 500,000 operations to fix or replace heart valves damaged due to birth defect, infection and age related degeneration. Two types of replacement valves are available, mechanical heart valves (MHVs) which require lifetime anticoagulation and bioprosthetic heart valves (BHVs) made from biological tissues, typically human or porcine heart valve leaflets or animal pericardium. BHVs are preferred in older patients (>60 years) where they are more durable. Patients under 60 generally receive MHVs due to rapid age-dependent BHV degeneration. In patents under 35 years of age up to 100% structural valve deterioration (SVD) occurs within 5 years. More durable BHVs would advance the standard of care by eliminating the need for anticoagulation in younger patients and extending access to this therapy to more patients.
BHV degeneration, epitomised by calcification and non-calcific structural failure, is attributed to mechanical stress, plasma calcium binding to glutaraldehyde or cellular lipids, and immune injury. The contribution of these processes may differ across patient age and disease groups. Current anti-calcification treatments include additional lipid extraction procedures designed to remove potential calcium nucleation sites. These procedures and alternative fixatives have not enhanced BHV durability in older patients or permitted their routine use in younger patients. This suggests that immune mediated BHV injury mechanisms may have a disproportionate contribution to BHV degeneration in young adults (35 – 60 years) who have a more active immune system compared to older patients.
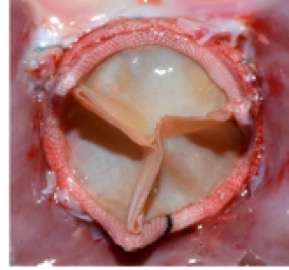
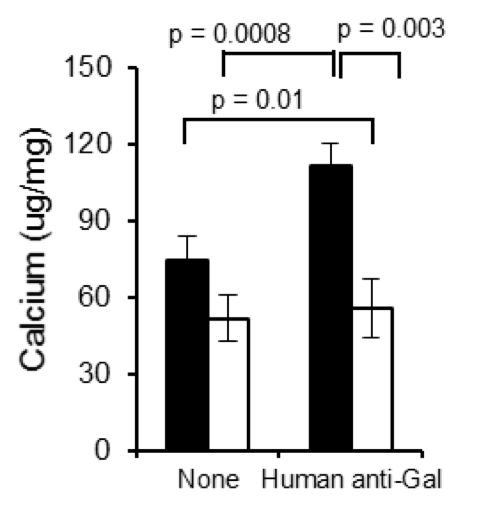 The group of Prof McGregor has identified an antibody-mediated inflammatory process involving the dominant xenogeneic antigen galactose alpha 1,3 galactose (Gal) and universally present human anti-Gal antibody which enhances calcification of fixed Gal-positive (GT+) bioprosthetic materials. Evidence indicates that this mechanism may significantly contribute to age-dependent calcification in young patients who have higher affinity anti-Gal antibody and are strongly sensitized to Gal after BHV implantation. Genetically modified pigs which do not make the Gal antigen (GTKO) can provide a new source of material for BHV manufacture. Fix GTKO pig pericardium does not bind anti-Gal antibody and is resistant to anti-Gal induced calcification. BHVs made from GTKO tissue will have lower antigenicity and may be suitable for use in younger patients.
The group of Prof McGregor has identified an antibody-mediated inflammatory process involving the dominant xenogeneic antigen galactose alpha 1,3 galactose (Gal) and universally present human anti-Gal antibody which enhances calcification of fixed Gal-positive (GT+) bioprosthetic materials. Evidence indicates that this mechanism may significantly contribute to age-dependent calcification in young patients who have higher affinity anti-Gal antibody and are strongly sensitized to Gal after BHV implantation. Genetically modified pigs which do not make the Gal antigen (GTKO) can provide a new source of material for BHV manufacture. Fix GTKO pig pericardium does not bind anti-Gal antibody and is resistant to anti-Gal induced calcification. BHVs made from GTKO tissue will have lower antigenicity and may be suitable for use in younger patients.
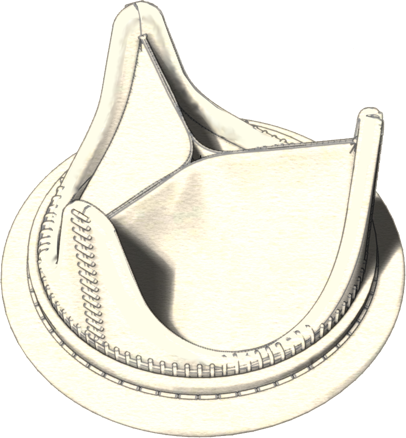 Now, partnering with Prof McGregor and his team, we are developing a bioprosthetic heart valve which resists calcification, broadening the patient population and improve the quality of life of recipients who receive this improved therapy.
Now, partnering with Prof McGregor and his team, we are developing a bioprosthetic heart valve which resists calcification, broadening the patient population and improve the quality of life of recipients who receive this improved therapy.
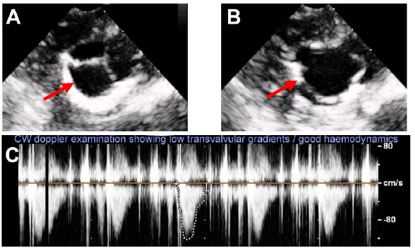
We have already verified that both GTKO and standard pig tissue have the same general morphology and collagen content, and uniaxial stress and suture retention testing has shown that the tissues are equivalent in tensile strength. Bioprosthetic valve prototypes based on the two tissues, designed and developed in our team, have indicated that GTKO and standard pig tissue provide similar valve durability and failure mode. In vitro and in vivo acute assessment has shown for both tissues excellent and equivalent hydrodynamics (as per the standard ISO 5840:2015). Durability tests are ongoing, and preliminary results are very encouraging.
 Close
Close


 The group of Prof McGregor has identified an antibody-mediated inflammatory process involving the dominant xenogeneic antigen galactose alpha 1,3 galactose (Gal) and universally present human anti-Gal antibody which enhances calcification of fixed Gal-positive (GT+) bioprosthetic materials. Evidence indicates that this mechanism may significantly contribute to age-dependent calcification in young patients who have higher affinity anti-Gal antibody and are strongly sensitized to Gal after BHV implantation. Genetically modified pigs which do not make the Gal antigen (GTKO) can provide a new source of material for BHV manufacture.
The group of Prof McGregor has identified an antibody-mediated inflammatory process involving the dominant xenogeneic antigen galactose alpha 1,3 galactose (Gal) and universally present human anti-Gal antibody which enhances calcification of fixed Gal-positive (GT+) bioprosthetic materials. Evidence indicates that this mechanism may significantly contribute to age-dependent calcification in young patients who have higher affinity anti-Gal antibody and are strongly sensitized to Gal after BHV implantation. Genetically modified pigs which do not make the Gal antigen (GTKO) can provide a new source of material for BHV manufacture. Now, partnering with Prof McGregor and his team, we are developing a bioprosthetic heart valve which resists calcification, broadening the patient population and improve the quality of life of recipients who receive this improved therapy.
Now, partnering with Prof McGregor and his team, we are developing a bioprosthetic heart valve which resists calcification, broadening the patient population and improve the quality of life of recipients who receive this improved therapy.