GEMINI - Transcatheter Mitral Valve
Mitral regurgitation (MR) is one of the most common forms of heart valve disorder, occurring when blood leaks from the left ventricle into the left atrium. If untreated, MR results into increased heart load and failure risk. It is estimated that symptomatic MR affects over four million Europeans and, due to the continuous ageing of our population, the number of cases is expected to rise significantly in the future.
In recent years, the need for less invasive therapeutic approaches has led to the development of a number of reconstructive percutaneous treatments for MR. However the available approaches mainly contribute to alleviate the symptoms and are suitable for very specific forms of mitral valve disease and anatomic subset. The possibility to perform a complete percutaneous mitral valve replacement still represents an unmet need which would provide enormous advance for a vast patients population.
Percutaneous and transcatheter valve replacement have already been successfully applied in the treatment of pulmonary and aortic valves. However, despite the relevance of the described clinical need, these approaches have not been successfully transferred to the mitral valve, due to the technical challenges associated with the geometry of the anatomical site and the more severe loading conditions.
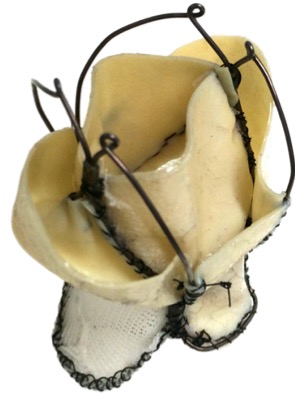 The objective of this project is to expand the benefits of transcatheter valve replacement to the mitral valve, developing a novel prosthetic device suitable for this application. Our valve is designed to match the anatomy and function of the native mitral valve, and consists of a self expanding wireframe structure made from superelastic NiTi alloy, supporting two leaflets and a sealing component made from xenograft pericardium. The frame was designed to provide adequate matching to the D-shape mitral valve anatomy, and optimised numerically to minimise the stresses during crimping. Leaflets were designed to minimise the stress during systole and provide sufficient length to guarantee proper coaptation at different geometries of the host anatomy. A skirt made from a polymeric mesh was included to gently distribute the anchoring force over the annulus.
The objective of this project is to expand the benefits of transcatheter valve replacement to the mitral valve, developing a novel prosthetic device suitable for this application. Our valve is designed to match the anatomy and function of the native mitral valve, and consists of a self expanding wireframe structure made from superelastic NiTi alloy, supporting two leaflets and a sealing component made from xenograft pericardium. The frame was designed to provide adequate matching to the D-shape mitral valve anatomy, and optimised numerically to minimise the stresses during crimping. Leaflets were designed to minimise the stress during systole and provide sufficient length to guarantee proper coaptation at different geometries of the host anatomy. A skirt made from a polymeric mesh was included to gently distribute the anchoring force over the annulus.
In vitro assessment of the valve prototypes have confirmed a good anchoring of the valve, and compliance with the hydrodynamic requirements in the international standard ISO5840:2015-3, demonstration its suitability to effectively replace the mitral valve function.
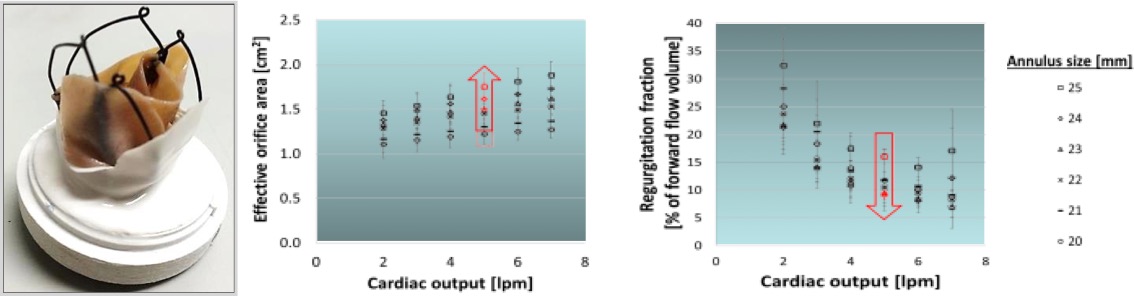
This novel solution could make possible to quickly perform mitral valve functional replacements in cardiac catheterisation laboratories and with shorter recovery time, thus reducing dramatically the costs to ensure proper treatment to the population. The reduction in costs and resources would contribute to enable valve replacement operations in many parts of the world where they are currently limited or not available, due to the resource-intensive requirements of surgery.
 Close
Close


 The objective of this project is to expand the benefits of transcatheter valve replacement to the mitral valve, developing a novel prosthetic device suitable for this application.
The objective of this project is to expand the benefits of transcatheter valve replacement to the mitral valve, developing a novel prosthetic device suitable for this application.









